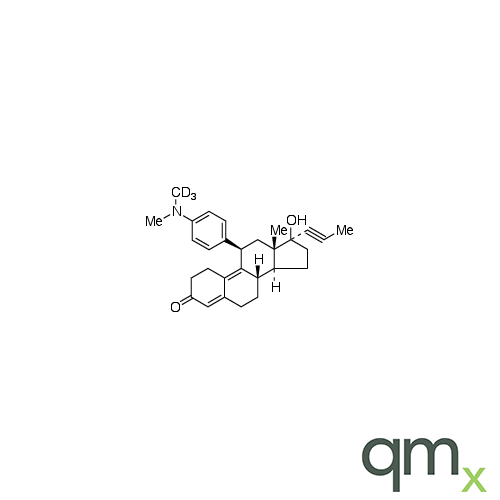
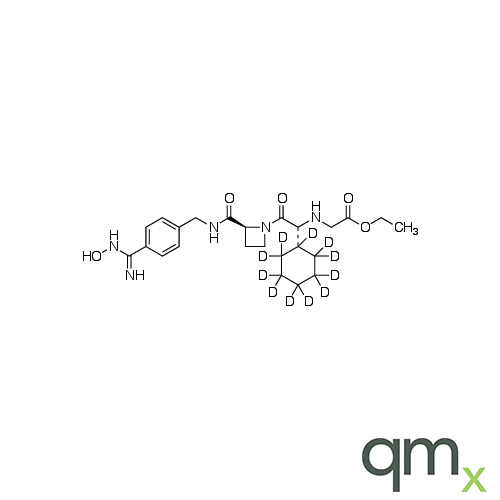
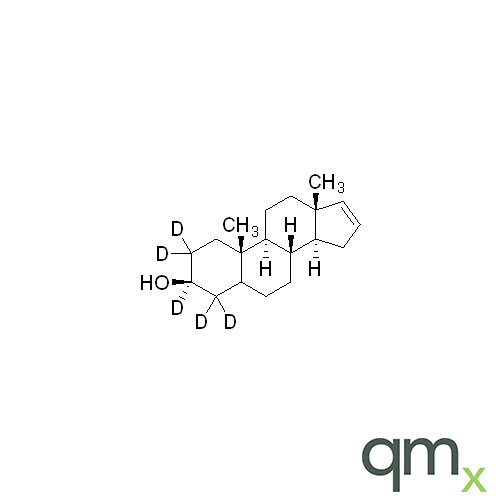
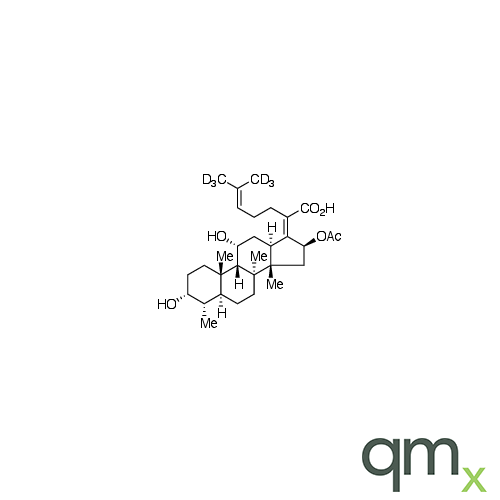
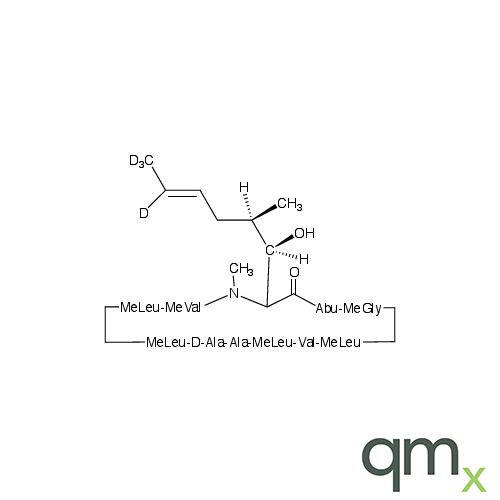
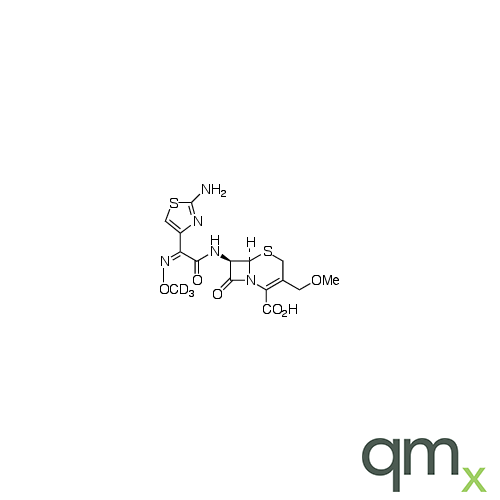
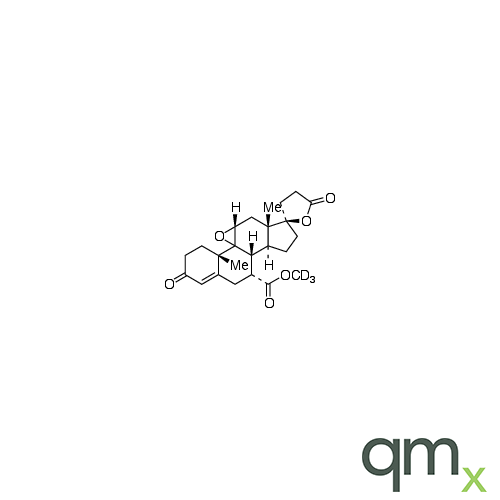
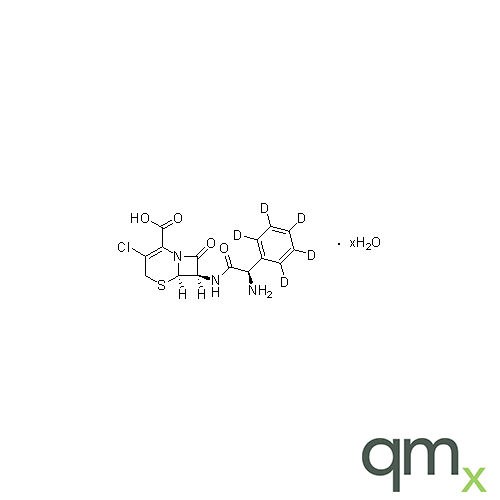
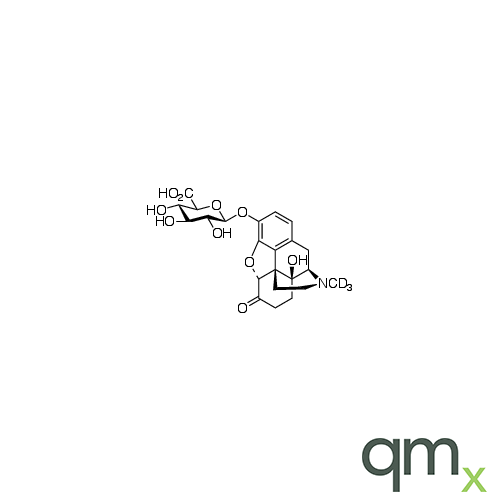
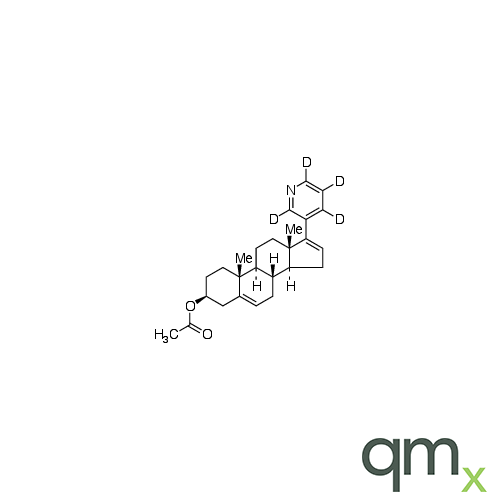
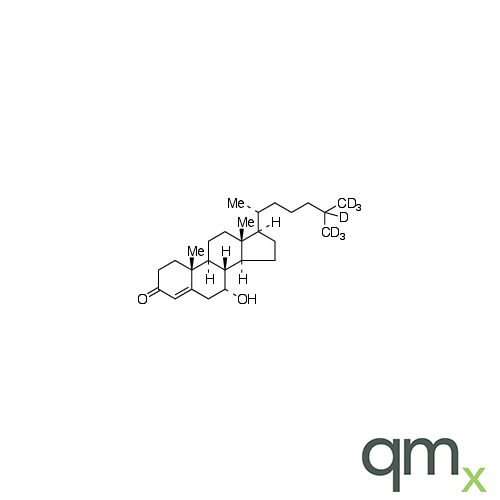
Pharmaceuticals
We offer an extensive range of isotopically labelled reference standards for pharmaceutical and veterinary research, analysis and screening. The range includes isotopically labelled reference standards of Active pharmaceutical ingredients, Metabolite and impurity products, Degradation products and process impurities, Inhibitor and receptor compounds, Genotoxic impurities and related substances for pharmacopeial updates.
Please don’t hesitate to contact us if you are unable to find the stable isotope that you need and we'll be delighted to check on its availability.